ASTM F2101 The Test Procedure of Bacteria Filtration Efficiency Tester (BFE)
1.The aerosol challenge apparatus is outlined in Fig. 1.

2.Deliver the challenge to the nebulizer using a peristaltic or syringe pump. Connect tubing to nebulizer and peristaltic pump and into the challenge suspension; purge tubing and
nebulizer of air bubbles.
3.Perform a positive control run without a test specimen clamped into the test system to determine the number of viable aerosol particles being generated. The mean particle size
(MPS) of the aerosol will also be calculated from the results of these positive control plates.
4.Initiate the aerosol challenge by turning on the air pressure and pump connected to the nebulizer.
5.Immediately begin sampling the aerosol using the cascade impactor. Adjust the flow rate through the cascade impactor to 28.3 L/m.
6.Time the challenge suspension to be delivered to the nebulizer for 1 min.
7.Time the air pressure and cascade impactor to run for 2 min.
8.At the conclusion of the positive control run, remove plates from the cascade impactor. Label each plate with the corresponding stage number.
9.Place new agar plates into the cascade impactor and clamp the test specimen into the top of the cascade impactor, with either the inside or outside oriented toward the challenge
as intended.
10.Initiate the aerosol challenge as outlined above.
11.Repeat the challenge procedure for each test specimen.
12.Repeat a positive control sample after completion of the test sample set.
13.Perform a negative control sample by collecting a 2-min sample of air from the aerosol chamber. No bacterial challenge should be pumped into the nebulizer during the collection of the negative control sample.
14.Incubate agar plates at 37 ±2 °C for 48±4 h.
15.Count each of the six-stage plates of the cascade impactor.
16.Total the counts from each of the six plates for the test specimens and positive controls, as specified by the manufacturer of the cascade impactor. The filtration efficiency percentages are calculated using the following equation:
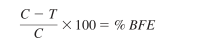
where:
C = average plate count total for test controls, and
T = plate count total for test sample.
17.Calculate the mean particle size using the specification of the manufacturer of the cascade impactor. The mean particle size of the bacterial aerosol shall be maintained at 3.0 µm ±0.3 µm.